Risk-Benefit Calculator
Understand Your Medication's Benefits
Compare absolute risk reduction (actual change) with relative risk reduction (percentage change) to see if a drug's benefits are meaningful.
When you pick up a new prescription, the label that comes with it isn’t just a list of side effects. It’s a carefully written summary of whether the drug’s benefits are worth the risks - and most patients have no idea how to read it.
What FDA Risk-Benefit Statements Really Mean
The U.S. Food and Drug Administration (FDA) doesn’t just approve drugs because they work. They approve them only if the benefits clearly outweigh the risks. That’s called a risk-benefit assessment. But what does that actually look like on the label you hold in your hand?
Behind every FDA-approved drug is a detailed analysis. Reviewers look at clinical trial data, real-world use, how severe the condition is, and what other treatments exist. For example, a cancer drug might cause serious nausea and hair loss - but if it extends life by months when nothing else works, the FDA considers that a fair trade. For a medication used to treat mild anxiety, the same side effects might be unacceptable.
Since 2021, the FDA has required sponsors to use a structured framework called the Benefit-Risk Framework to organize this information. It’s not just about listing side effects. It’s about explaining why, for the intended patient group, the benefits are expected to be greater than the risks. And that’s where things get confusing.
Where to Find the Real Information in the Label
You won’t find a simple “Benefits vs. Risks” chart on the first page. The key sections are buried in the prescribing information:
- Section 5: Contraindications - Tells you when NOT to take the drug. This is where serious risks are flagged, like dangerous interactions or allergies.
- Section 6: Adverse Reactions - Lists side effects, but often without context. Did 1 in 10 people get headaches? Or 1 in 1,000? The label might not say.
- Section 14: Clinical Studies - This is the goldmine. Here, you’ll find actual numbers: “Reduced risk of heart attack by 27%” or “38% fewer deaths compared to placebo.” These are the facts that matter.
- Highlights Section - A quick summary at the top. If the drug has a boxed warning (the FDA’s strongest alert), it’s here.
But here’s the problem: most labels use medical jargon. Phrases like “statistically significant reduction in events” mean nothing to someone without a science background. A 2022 survey found only 22% of patients felt confident understanding this information. For those with low health literacy, that number drops to 9%.
Why Numbers Can Be Misleading
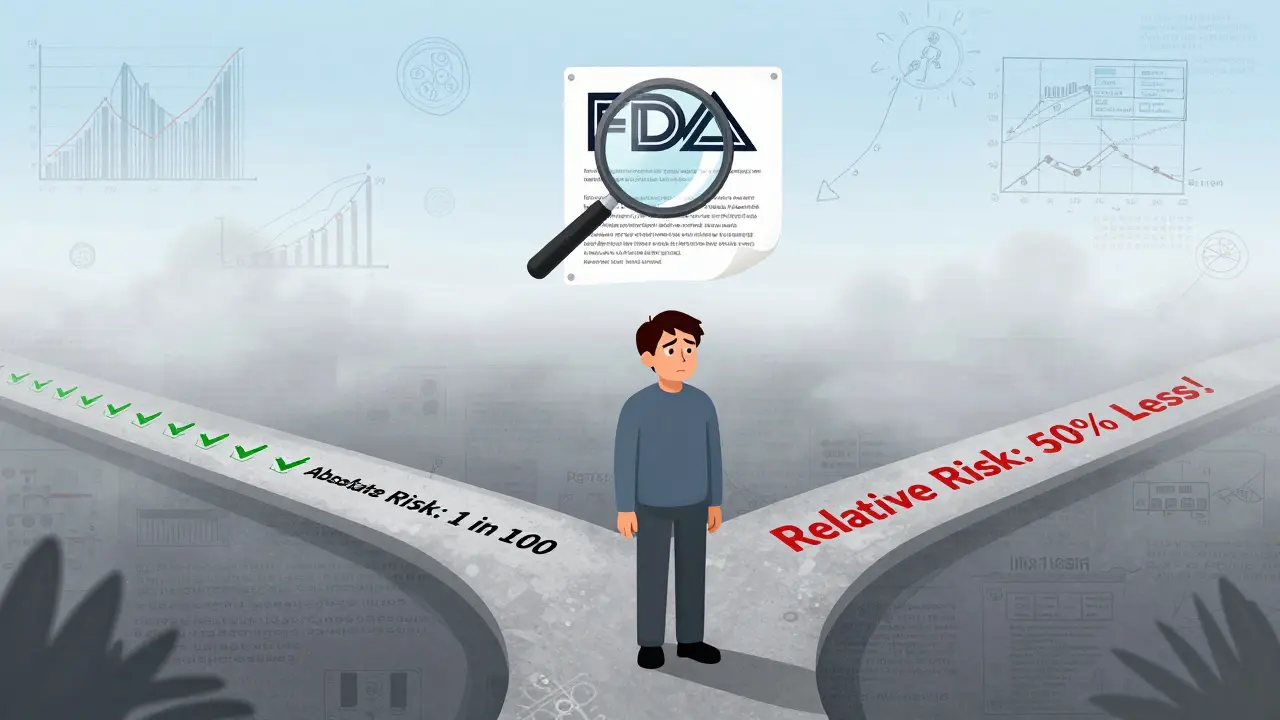
Drug companies often report relative risk reduction - like “reduced risk by 50%.” Sounds impressive, right? But if the original risk was 2 in 1,000, a 50% reduction means it’s now 1 in 1,000. That’s not a huge change.
Dr. Thomas Fleming from the University of Washington pointed out this problem years ago: relative risk numbers make small benefits look big. The FDA now encourages absolute risk numbers - the actual change in likelihood - but many labels still don’t use them.
Take Jardiance, a diabetes drug. Its label says: “In adults with type 2 diabetes and heart disease, Jardiance reduced the risk of cardiovascular death by 38% (10.5% with placebo vs. 6.5% with Jardiance).” That’s clear. You see the real numbers. That’s rare. Most labels don’t do this.
What the FDA Is Doing to Fix It
The FDA knows patients are struggling. In 2023, they launched a pilot program requiring six new cancer drugs to include a Patient Benefit-Risk Summary - written at a 6th-grade reading level, with simple visuals.
These summaries answer three questions:
- What does this drug do?
- What are the most common side effects?
- How does it compare to other options?
They’re also testing new icons - tiny pictures that show benefit vs. risk. One icon might show a large green arrow up for benefit, and a small red arrow down for risk. Another might show a scale with weights. These are being tested in 12 clinics with over 1,500 patients.
By 2025, the FDA plans to require standardized benefit-risk metrics for major drug categories. That means more consistent, easier-to-compare data across drugs. And by 2026, nearly half of new drug labels could include visual summaries - up from just 8% in 2022.
How to Read a Label Like a Pro
You don’t need a medical degree to understand your prescription. Here’s how to cut through the noise:
- Look for absolute numbers - Ask: “Out of 100 people, how many actually benefited? How many had serious side effects?”
- Compare to alternatives - If your doctor says “this drug is better,” ask: “How is it better than the other options?”
- Check the Highlights - The boxed warning is there for a reason. If it says “risk of liver damage,” take it seriously.
- Use the Clinical Studies section - Skip the jargon. Look for percentages, numbers, and comparisons.
- Ask your pharmacist - They’re trained to translate these labels. Ask: “Can you explain the benefit and risk in plain terms?”
Some labels are getting better. The FDA’s own Transparency Initiative has pushed companies to simplify language. But progress is slow. Only 17% of new drugs approved in 2022 had any kind of visual benefit-risk summary.
Why This Matters for You
Medication decisions aren’t just about science - they’re personal. One person might accept a 1 in 50 risk of severe rash if it means they can walk without pain. Another might refuse it. The FDA’s framework tries to account for both.
But right now, the system is designed for regulators, not patients. You’re expected to understand complex data without tools or training. That’s unfair.
When you understand the real benefit-risk picture, you can have better conversations with your doctor. You can ask: “Is this worth it for me?” instead of just taking what’s handed to you.
The FDA is moving in the right direction. Visuals, plain language, and patient input are finally being taken seriously. But until those changes become standard, you need to be your own advocate.
What’s Still Missing
Even with improvements, gaps remain:
- Psychiatric drugs - Benefits like “feeling calmer” are hard to measure. Risks like weight gain or sexual dysfunction are often underreported.
- Long-term risks - Many side effects show up after years. Labels rarely address this.
- Real-world data - Clinical trials are controlled. Real life isn’t. Labels don’t always reflect how people actually use the drug.
- Cost and access - The FDA doesn’t consider price. A drug might have great benefits, but if you can’t afford it, the risk-benefit calculation changes.
That’s why patient advocacy groups are pushing for more transparency. They want labels to include not just what the drug does, but what it costs, how it affects daily life, and how it compares to non-drug options like therapy or lifestyle changes.
Until then, the best tool you have is knowledge. Don’t just read the label - question it. Ask for help. Demand clarity.
What’s the difference between relative risk and absolute risk in FDA labels?
Relative risk compares how much a drug reduces risk compared to a placebo - like “reduces risk by 50%.” Absolute risk shows the actual change: “Out of 100 people, 2 had a heart attack without the drug; 1 had it with the drug.” Absolute risk tells you the real impact. Many labels use relative risk because it sounds better, but absolute risk is what matters for your decision.
Why do FDA labels use so much medical jargon?
Historically, labels were written for doctors, not patients. The FDA now encourages plain language, but many drug companies still use technical terms to avoid liability or because they’re used to old formats. The 2023 pilot program is changing that - new labels for cancer drugs are being required to use 6th-grade reading levels. But it’s still not the norm.
Can I trust the side effects listed on the label?
Yes, but with context. The FDA requires all side effects seen in clinical trials to be listed, even rare ones. But that doesn’t mean you’ll get them. A side effect listed as “1 in 1,000” means it’s unlikely. What’s more important is whether the side effect is serious and how often it happens. Always check if the label says “common,” “uncommon,” or “rare.”
Do FDA labels include information about non-drug alternatives?
No. FDA labels only compare the drug to placebo or other drugs tested in trials. They don’t mention lifestyle changes, therapy, or surgery - even if those might be better options for you. That’s why you need to talk to your doctor about all possible treatments, not just the prescription.
What should I do if I don’t understand my drug’s label?
Ask your pharmacist or doctor to explain it in plain language. You can also look up the full prescribing information on the FDA’s website (fda.gov/drugs) and search for the drug name. Focus on the Highlights section and Section 14 (Clinical Studies). Look for numbers, not just words. And don’t be afraid to ask: “Can you tell me in simple terms whether this drug is right for me?”
What Comes Next
The future of drug labels is simpler, clearer, and more visual. By 2026, most new drugs - especially for serious conditions - will include easy-to-read summaries with icons and charts. The FDA is also requiring patient input before approval, meaning your voice will shape how risks and benefits are described.
But until then, you’re not powerless. You can learn to read between the lines. You can ask for better explanations. And you can push for clarity - because your health shouldn’t depend on decoding medical code.

Comments (11)
Ambrose Curtis
man i read my last prescription label like it was a novel and still missed half the stuff. they say 'statistically significant' like it means something to me. i just wanna know if i'm gonna puke or not. why can't they just say '1 in 5 people get nausea' instead of all this fancy crap?
Linda O'neil
Finally someone breaks this down without jargon. I’m a nurse and even I get overwhelmed by these labels. The clinical studies section is where the truth lives-skip the highlights and go straight to the numbers. If your doctor can’t explain the absolute risk, ask for a pharmacist. They’re the real MVPs here.
Robert Cardoso
You’re all missing the fundamental flaw here. The FDA doesn’t care about patient understanding-they care about liability mitigation. The 'benefit-risk framework' is a legal shield, not a patient tool. The jargon exists because if you understand it, you might sue them when the side effects hit. This isn’t transparency-it’s obfuscation with a FDA stamp.
James Dwyer
This is actually really hopeful. I used to just swallow pills without thinking. Now I check the clinical studies section like it’s a treasure map. Finding that 38% number for Jardiance made me feel like I actually understood something for once. Small wins, right?
Rose Palmer
It is imperative that patients be empowered with accessible, evidence-based information regarding pharmacological interventions. The current regulatory framework, while scientifically rigorous, remains linguistically inaccessible to a significant portion of the population. Standardized visual metrics and plain-language summaries are not merely beneficial-they are ethically obligatory. I commend the FDA’s 2023 pilot program as a necessary step toward health equity.
Howard Esakov
Of course the FDA is dragging its feet. These labels were designed for physicians who attended Ivy League med schools, not the average person who thinks 'statistical significance' is a type of coffee. If you can’t read a label, maybe you shouldn’t be taking prescription drugs. It’s not the system’s fault-it’s yours for not being educated enough.
Timothy Davis
Relative risk is misleading? Please. Absolute risk is meaningless without context. A 50% reduction sounds scary until you realize it went from 0.002% to 0.001%. The FDA uses relative risk because it’s accurate in context. People who complain about this don’t understand statistics-they just want to feel safe, not informed.
Lexi Karuzis
They’re hiding something. Why do they only test on healthy volunteers? Why aren’t the long-term effects of antidepressants on fertility included? And why do the same drugs get approved in Europe but banned here? The FDA is bought. The 'visual summaries' are just glitter on a toxic pill. They want you to feel better about taking poison.
Brittany Fiddes
Oh, so now we’re supposed to trust American drug labels? In the UK, we get real patient leaflets-not this corporate propaganda. You think this '6th-grade reading level' is progress? We’ve had plain-language summaries since 2015. You Americans still think 'FDA-approved' means 'safe'-it means 'profitable.'
Colin Pierce
My mom didn’t understand her blood pressure med until I sat down with her and showed her Section 14. We looked up the actual numbers-she went from scared to empowered. You don’t need a degree. You just need someone to sit with you and point at the numbers. Ask your pharmacist. They won’t judge you.
Mark Alan
THEY’RE LYING TO US!!! 😡💊
Why does every drug have a 'rare' side effect that kills 3 people a year? But they still sell it? 🤬
It’s not about risk vs benefit-it’s about stock prices. 📉
Read the fine print? Nah. Burn the label. 🚫
My cousin took this drug and lost his memory. Now he can’t remember his own name. 🤯
They don’t care. They just want your money. 💸